What is chelated fertilizer
؟What Is Chelated Fertilizer
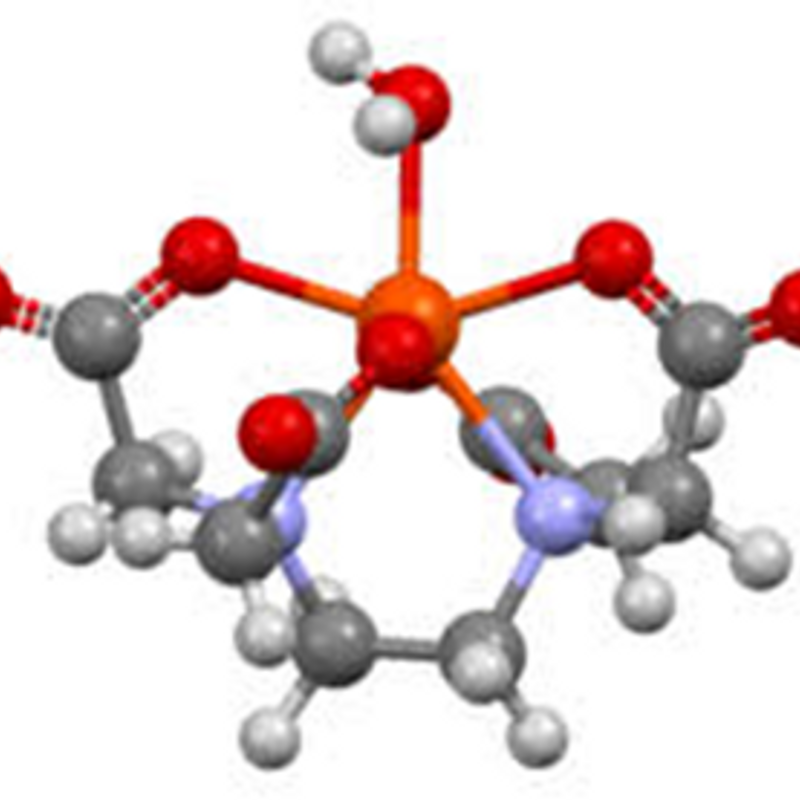
Chelated fertilizer is a type of fertilizer that contains nutrients in the form of chelates. Chelates are compounds in which a nutrient, typically a metal ion such as iron, zinc, copper, or manganese, is bounded to an organic molecule (the chelating agent). This binding forms a stable, water-soluble complex that protects the nutrient from reacting with other compounds in the soil. Chelated fertilizers are specialized formulations that contain essential micronutrients bound to organic molecules called chelators or ligands. This binding process increases the stability and availability of these nutrients. Chelated fertilizers are specialized fertilizers in which essential micronutrients (such as iron, zinc, copper, manganese, etc.) are bounded to organic molecules known as chelating agents. Chelating agents are ligands that are capable of donating two or more electron pairs to a metal ion, producing stable ring complexes called chelates.
Common chelated micronutrients include iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), boron (B), and molybdenum (Mo). Various chelating agents such as EDTA, DTPA, and EDDHA affect the stability and availability of the micronutrients. Chelated fertilizers can be applied via foliar application, or through irrigation systems
What Are the Benefits of Chelated Fertilizers
Advantages of Chelated Fertilizers
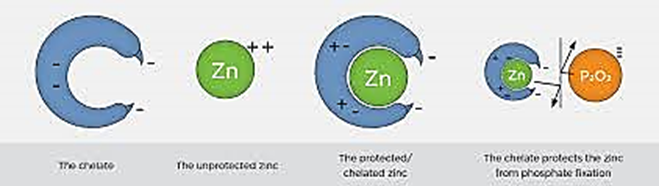
- Increased Nutrient Availability:Chelated fertilizers enhance the availability of micronutrients to plants. This feature is especially beneficial in soils with high pH. During the chelation process, micronutrients are surrounded by organic molecules, which facilitates their uptake by plant roots. This leads to more efficient nutrients absorption, resulting in faster growth and improved plant health
- Greater stability and plant health: Chelated micronutrients are more stable in crop conditions than conventional fertilizers and release nutrients slowly over time
- Correction of Micronutrients Deficiency: Chelated fertilizers are particularly effective in correcting deficiencies of essential micronutrients such as iron, zinc, and manganese, especially in alkalin soils where these nutrients are not readily available
- Improving Soil Efficiency: Chelated fertilizers remain stable across a wide range of soil pH levels, ensuring nutrients availability even in alkaline soils
- Better Plant Health and Growth: By supplying essential nutrients, chelated fertilizers support healthier plant growth, development of a stronger root system, and increased resistance to stresses and diseases
- Lower Application Rates: Because chelated fertilizers are more efficient, they often require lower application rates compared to non-chelated fertilizers, reducing costs and minimizing the risk of over-fertilization
- Suitable for Foliar Application: Nutrients in chelated fertilizers are easily absorbed through leaves, making them ideal for foliar sprays that can quickly address nutrients deficiencies
- Environmentally Friendly: By improving nutrients uptake efficiency, chelated fertilizers reduce the risk of nutrient leaching into groundwater, making them a more sustainable and environmentally friendly option
Characteristics of Chelated Fertilizers
FormationofStableComplexes
Chelated fertilizers form stable and water-soluble complexes between metal ions (such as iron, zinc, copper, manganese) and organic molecules called chelating agents (e.g., EDTA, DTPA, EDDHA)
Stability in a Extende Range of pH Levels: Chelated nutrients remain available over a wide range of soil pH levels, including alkaline soils where many micronutrients typically become insoluble and unavailable to plants
Improving Nutrient Mobility: The chelation process increases the mobility of nutrients in the soil, allowing these nutrients to become more readily available to the plant
High Bioavailability:Chelated nutrients are easily absorbed by plant roots or foliage, ensuring efficient uptake and utilization by plants
Reducing Nutrient Fixation: The chelation process prevents nutrients from binding to soil particles or forming insoluble compounds, reducing nutrients fixation and increasing their availability
Targeted Nutrient Delivery: Chelated fertilizers are especially effective for delivering micronutrients, which are required in small amounts but are essential for plant growth and development
Compatibility: Chelated fertilizers are compatible with most fertilizers and agricultural chemicals and can be applied through various methods, including irrigation and foliar spraying
These features make chelated fertilizers a valuable tool for correcting nutrients deficiencies, improving crop performance, and ensuring plant health, especially under adverse soil conditions
How Do Chelated Fertilizers Work
Chelated fertilizers enhance nutrient uptake through various mechanisms
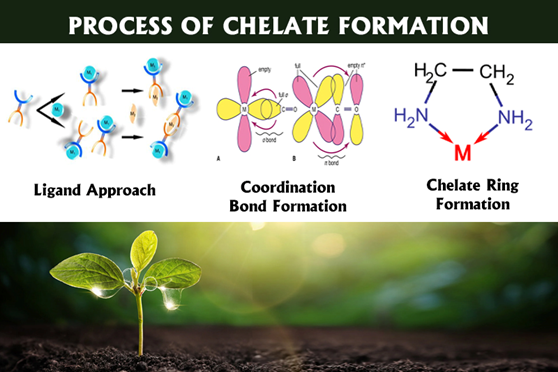
The mechanism of action of chelated fertilizers is based on the chemical bonding of essential micronutrient ions with organic molecules called chelating agents or ligands. This creates stable, water-soluble complexes that protect the nutrient ions from adverse reactions in the soil, such as oxidation, precipitation, or stabilization. Chelated fertilizers work by binding essential micronutrient metal ions, such as iron, zinc, and manganese, to organic molecules called chelating agents. This binding forms stable complexes called chelates that prevent the nutrients from reacting with other elements in the soil and becoming unavailable. Plants can easily absorb the chelated or chelated micronutrient complex, resulting in more efficient absorption of the micronutrients.The organic ligand around the chelated micronutrient can penetrate the waxy layer of crop leaves and enhance absorption during foliar application. When the nutrient is released near the root, the chelating agent can bind to another nutrient ion, continuing the cycle and maximizing nutrient use efficiency
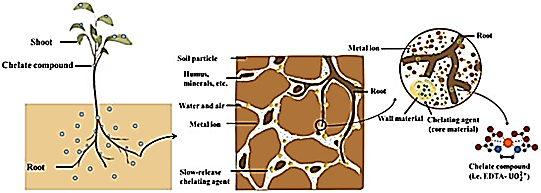
Types of chelates
- Based on the Nature of the Chelate
Organic Chelates such as Aminoacids:Organic chelates such as amino acids: Amino acid chelates are complexes that form when amino acids bind to metal ions through a process called chelation. In this process, the amino acid acts as a ligand and uses its amino group (-NH2) and carboxyl group (-COOH) to form coordination bonds with the metal ion, creating a stable ring structure around the metal. This increases the stability of the metal ions. In agriculture, amino acid chelates help to make essential nutrients such as iron, manganese, zinc, calcium, copper, and magnesium more available to plants by preventing them from becoming insoluble in the soil. They also reduce toxicity and improve nutrient uptake
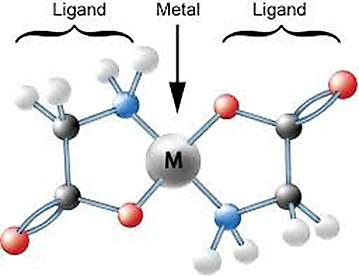
Chemical Chelates such as EDTA: EDTA (ethylenediaminetetraacetic acid) is a widely used chelating agent that forms stable complexes with metal ions through coordination bonds. It effectively “captures” and holds metal ions such as calcium, iron, manganese, copper, and cobalt by binding through its four carboxylate groups and two amine groups, forming soluble complexes (chelates) that prevent micronutrient reactions with other compounds
Based on Dentate Number
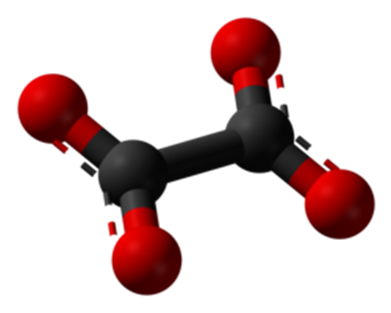
Bidentate chelates: Bidentate chelates are coordination compounds formed when a metal ion is bound to two different ligands. These compounds usually contain chelating agents that form ring structures with the metal. A "bidentate chelate" is a bidentate ligand, a type of molecule that can bind to a central metal atom or ion at two different points. Ligands with two attachment points form chelate rings when bound to metal ions, increasing the stability of the complex through the "chelate effect." This effect is attributed to the thermodynamic stability resulting from ring formation and makes chelates generally more stable than similar non-chelated compounds
Tetradentate Chelates: Tetradentate chelates refer to coordination compounds in which a central metal ion is bounded to a ligand through four donor atoms or groups, forming a complex structure that typically includes a ring. These ligands are described as tetradentate, meaning they have four “teeth” or binding sites attaching to the metal ion.This multidentate binding, known as the chelate effect, results in greater stability of the chelate complex— a phenomenon where chelating ligands form more stable complexes compared to similar monodentate ligands.Tetradentate chelates are less common than bidentate or tridentate ones, but they are notable for their improved binding strength and structural stability around the metal center, often creating stable ring systems
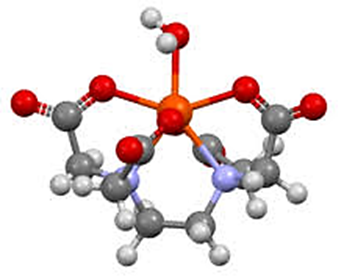
HexadentateChelates
A hexadentate ligand is a multidentate ligand with six coordination sites, allowing it to bind to a central metal ion through six atoms. This bonding creates a cage-like structure around the metal, which increases stability and protects the metal ion from interactions with solvents or other molecules.One well-known hexadentate ligand is EDTA (ethylenediaminetetraacetic acid), which contains four carboxylate groups and two amine groups as donor atoms. EDTA effectively forms stable chelates with various metal ions
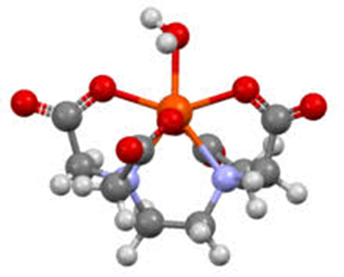
What Characteristics Must Metalic Ion Have to Form Chelates
Ability to Form Coordinate Covalent (Dative) Bonds: The metalic onion must be capable of forming coordinate covalent bonds, where it acts as a Lewis acid (electron pair acceptor). This is typically seen in metal ions that have vacant orbitals available to accept electron pairs from the chelating agent
Appropriate Charge and Size: The metalic ion should have a suitable ionic charge and size to allow stable coordination with the chelating ligand. For example, transition metals often have charges of 2+ or 3+, which fit well within the coordination sphere of chelating ligands
Electronegativity: The metalic ion should has an electronegativity that enables it to attract and hold the electron pairs donated by the chelating agent
Stability Constant: The metalic ion must form complexes with high stability constants, meaning the chelate complex is thermodynamically stable and does not readily dissociate
Conclusion
Chelated fertilizers are an effective and versatile solution for optimizing micronutrients nutrition in agricultural crops, enabling better growth and productivity even in soils with limited conditions. Their ability to maintain nutrient availability in a wide range of environments makes them an essential tool in modern sustainable agricultural practices.





